
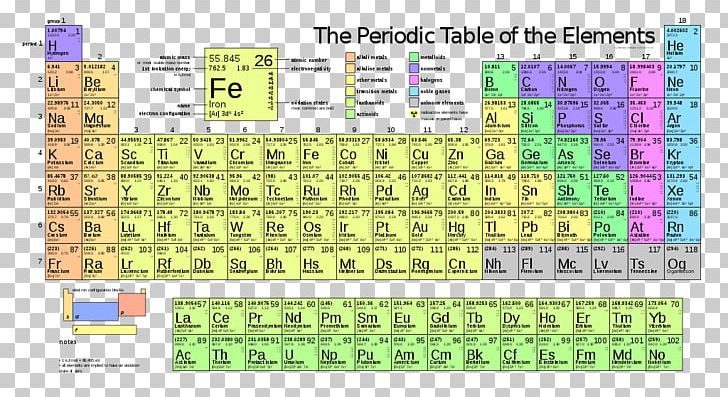
One group at University of California, Berkeley, led by the scientist, Glenn Seaborg, proceeded to make new elements. Then, as people started theorizing, making suggestions, scientists were saying, maybe we can synthesize elements we can make new elements and The transuranium elements were synthesized by several people. Later on when we got to the so called heavy elements, to the elements that have 92 protons or more, uranium, for example. So that was a very, very important consequence of putting the elements in this organized fashion that we call the periodic table. So the search was on to find them and to identify them and to characterize them. When the elements were arranged in this chart in the periodic table, it was easy to see right away that there are missing elements that have not been discovered yet, that have not been identified. Quirmbach: What sort of applications took off? It was not to serve any other purpose, but curiosity leads to discovery and discovery leads to application. It's about examining behavior, natural behavior, and trying to describe in words and language and in symbols, what the behavior pattern might be. You know, why is the sky blue? We make observations and try to make sense out of these observations and that's what science is all about. We ask questions, all kinds of questions. Shakhashiri: Human beings all of us are naturally curious. So they range from one, which is element hydrogen, all the way to element 118 which is the total number of elements in the periodic table today,Ĭhuck Quirmbach: Was he creating this and and the others that can certainly contributed to it, were they trying to serve some industry like cannon makers? That's the number of protons in an atom of that element. We learn the periodic table as the elements arranged according to the atomic number, which is an integer, a whole number.
/PeriodicTableSigFigBW-58b5c7f25f9b586046cae098.png)
Later on, this turned out to be not so correct, but that's how it is in science. He proposed, sometimes, you know, people say he discovered the pattern of similar behavior and arranged the elements, according to are their atomic weights. And it came to him that there possibly is a pattern of repeated resemblance of behavior of certain elements.
ELEMENTS TABLE WITH ATOMIC MASS AND ATOMIC NUMBER FULL
Read the full transcript of WUWM's Chuck Quirmbach and Bassam Shakhashiri's conversation here:īassam Shakhashiri: Mendeleev the Russian chemist was teaching a course and he was writing his lecture notes and tried to write an inorganic chemistry book about chemical behavior of substances. Nowadays, lead is often viewed as a contaminant in soil and water. Shakhashiri also notes how lead (symbol Pb) has a less popular image than in Mendeleev's day. Lithium is also used in the medical profession, for a variety of things." On modern relevance, and how uses of the elements change, Shakhashiri cites lithium (symbol Li), in the table: "Lithium batteries are very useful to us. "Dimitri Mendeleev, the Russian chemist, he proposed - sometimes people say he discovered - the pattern of similar behavior and arranged them," Shakhashiri explains. He says the table came about through a collaboration of a few scientists but that Dmitri Mendeleev properly gets much of the credit.

UW-Madison professor of chemistry Bassam Shakhashiri knows both the history of the table, and its modern relevance. It's considered the founding document of modern chemistry, one you may have studied in school. Maybe you've felt a certain chemistry with 2019 but don't know why? Maybe it's because this year marks the 150th anniversary of the Periodic Table of the Elements.


 0 kommentar(er)
0 kommentar(er)
